Abstract
Background: The prognostic role of cardiac biomarker NT-proBNP was evaluated in different conditions. In presence of a MGUS, an increased value of NT-proBNP could rise the suspicion of cardiac AL amyloidosis in absence of any other cardiologic disease. Our group recently showed that the addition of NT-proBNP to a simple frailty score based on performance status and age, had a prognostic impact in patients with newly diagnosed multiple myeloma (Milani et al AJH, 2016). Aim of our study was to determine the role of NT-proBNP, a well-established cardiovascular risk biomarker, as a prognostic marker in patients with MGUS in an unselected cohort of patients.
Methods: Patients were eligible for this retrospective study if they were seen at the Mayo Clinic, Rochester, MN with a diagnosis of MGUS during the interval between 1/1/2000 and 12/31/2000. As part of the Mayo Clinic registration, all patients underwent a complete clinical assessment of their past medical history and symptoms. Data from that first visit was abstracted and used to calculate a Charlson comorbidity index (CCI). We excluded all patients with a concomitant diagnosis of multiple myeloma, light-chain AL amyloidosis and other plasma cell dyscrasias. We excluded also all patients that had a concomitant diagnosis of any other hematologic malignancies. NT-proBNP concentration was measured in frozen sera collected within 30 days of diagnosis. NT-proBNP assay was run on the E170 Modular analyzer (Roche Diagnostics, Penzberg, Germany). We evaluated the prognostic role of NT-proBNP and these indices on OS using the Kaplan-Meier method.
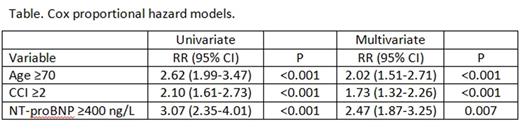
Results: Among the 394 consecutive patients satisfying entry criteria, median age was 70 years [interquartile range (IQR) 60-77], 16% were ≥80 years, and 55% were male. Fourteen patients had a subsequent diagnosis of a plasma cell dyscrasia (7 multiple myeloma, 3 smoldering MM, 1 AL amyloidosis, 2 Waldenstrom's macroglobulinemia and 1 LCDD), 7% had a creatinine ≥2 mg/dL, 33% had CCI ≥2, 29% had an history of hypertension and 6% had atrial fibrillation. The median value of NT-proBNP was 158 ng/L (IQR: 53-477 ng/L). Median overall survival (OS) was 7.6 years. The best cutoff of NT-proBNP predicting OS at two year was 400 ng/L (sensitivity: 78%, specificity: 63%; AUC=0.74) and distinguished two groups with different OS (median 146 vs. 36 months, P<0.001). In the subset of patients with age <70 years, NT-proBNP was able to distinguish two different groups (median survival 182 months vs. 35 months, P<0.001) as well as in the subset of patients older than 70 years of age (median survival 92 vs. 36 months, P<0.001). Variables predictive for OS are shown in the Table and included age, CCI, NT-proBNP. On multivariate analysis age≥70, CCI≥2 NT-proBNP≥400 ng/L were independent predictors of survival. Patients were assigned a score of 1 for each of these variables, creating stages I to IV with scores of 0 to 3 points, respectively. The median OS from diagnosis was 182, 91, 54 and 32 months, respectively.
Conclusions: In this unselected cohort of patients with MGUS, NT-proBNP was a useful predictor of survival independent of age and comorbidities score. The threshold of 400 ng/L is similar to the well-established cutoff for heart failure of 300 ng/L and pour proposed cutoff for frailty for patients affected by multiple myeloma. NT-proBNP is a widely available biomarker, could raise the suspicion of concomitant cardiac AL amyloidosis and we believe that could be added to the workup of patients with MGUS.
Gertz: Celgene, Novartis, Smith-Kline, Prothena, Ionis, Amgen: Honoraria; Millennium: Consultancy, Honoraria. Palladini: Celgene: Other: Travel grants; Prothena: Honoraria, Other: Travel grant; Jannsen-Cilag: Membership on an entity's Board of Directors or advisory committees. Kumar: Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding; Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Skyline: Honoraria. Dingli: Karyopharm Therapeutics: Research Funding; Janssen: Consultancy; Alexion Pharmaceuticals: Consultancy; Millenium: Consultancy; Takeda: Consultancy. Kapoor: Takeda, Celgene and Amgen: Research Funding. Dispenzieri: Celgene, Millenium, Pfizer, Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.